It is a known fact that we are living in the era of the Covid-19 pandemic. Our scientists and doctors are giving their best to defeat the coronavirus and their hard work paid off. We got a few vaccines against coronavirus. Have you ever wonder, How do they preserve these vaccines? And how are they distributing the vaccines throughout the world without destroying them? In this article, we are gonna tell you about the journey of a coronavirus vaccine from laboratory to vaccination centre.
How are vaccines made?
When a new pathogen such as a bacterium, virus, parasite or fungus enters the body, the body's immune system identifies the antigen (Antigens are proteins that are found on the surface of a pathogen) and starts produces antibodies against it.
The vaccine contains some weak or inactive portions of the virus that cause disease. It stimulates the body's 'immune system' to identify antigens (invasive viruses) and make antibodies against them in the body which help our body in fighting the external invasion.
In earlier times, vaccines were made by the traditional vaccine development method but now as technology developed scientists are trying to use new methods to develop vaccines. These new methods have also been used to make covid-19 vaccines.
Due to unprecedented scientific efforts at the global level, the world has been able to produce several vaccines of the coronavirus in record time. With the help of these vaccines, we will be able to protect those who are at risk due to this severe coronavirus pandemic.
Let us see how these vaccines are getting out from the scientists' labs in record time and reaching the public.
The vaccine's journey begins in the laboratory
For the first time, experts from all over the world started working together on different stages of vaccine development on a large scale. The result of this unprecedented global collaboration is that the development of the vaccine takes place in ten years but they were able to complete it within twelve months.
Many scientists who collaborated to develop the Covid-19 vaccine were among those who have been studying the virus since the SARS and MERS pandemic. Therefore, a strong foundation for preparing a vaccine to defeat the new coronavirus was already in place.
Scientists have studied this virus in detail so that they can detect that small element present in the virus, which activates our body's immune system or immunity is called the antigen.
Most of the vaccines contain a small blueprint of a virus or non-harmful parts of the virus. So the immune system of our body gets active and start producing antibodies by identifying the antigens present on the virus's surface. Scientists tested these antigens on a computer model in the lab so that their side effects could be monitored. Subsequently, the testing of these vaccines was started in humans.
Human test subjects
When these tests in the lab were completed, then these vaccines were applied to people in all the countries of the world, who were ready to test these vaccines on their own. These are called vaccine volunteers. The purpose of human trials of these vaccines was not only to ensure that the vaccines were safe for humans but also to find out how much vaccine dose would have to be given to avoid the virus.
It usually takes up to ten years to conduct such trials of a vaccine. However, multiple phase trials of Covid-19 vaccines were conducted simultaneously, allowing the vaccine to be used as quickly as possible. The results of a successful vaccine test were sent to institutions that certify the drugs as safe, and approve them to be used.
These organizations and their scientists closely analyzed the results of all the vaccine trials and the claims of their safety. Apart from this, the quality and effectiveness of these were also verified, so that the use of vaccines can be cleared.
To quickly complete the process of approving the use of the vaccine, the UK regulatory bodies used a system called 'Rolling Review' to wait for the vaccine trials to be completed and then analyze the data collected. During the trial, the vaccines were approved based on the analysis of the data being collected.
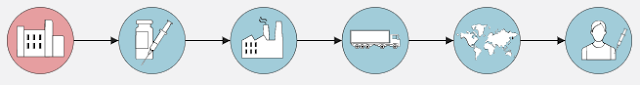
Large-scale Production of Vaccine
Normally only after reaching this stage — that is, when a drug is approved by the regulatory body — the large-scale production of drugs is started.
However, in the case of covid-19's vaccines, the ability to manufacture them on a large scale had already been developed before they were approved. That too when research on the vaccine was underway. The reason for this was that a large amount of investment was being made in preparing the vaccine.
The goal of developing the ability to make vaccines before they were approved was that when safe and effective vaccines were prepared and approved, so companies are ready for their earliest distribution.
During the process of making any vaccine, the active ingredients of the virus are mixed in large quantities with other elements such as stabilizers. During this time, the chemical is also added to the vaccine which is called 'Adjuvant'.
'Adjuvant' improves our immunity or immune system. These vaccines, prepared in large quantities, are quality tested before being packed in small disinfected vials and transported to their destination.
The vaccine is transported from one place to another by means of cold chains or mediums such as freezer vans and ice cooling refrigerators. When the manufacturer of the vaccine removes them from their manufactory, they are transported to a fixed destination using refrigerated trucks. This keeps the vaccine safe at a particular temperature.
Most traditional vaccines are protected at temperatures ranging from 2 to 8 degrees Celsius. However, there are many covid-19 vaccines that are stored at temperatures lower than this. For example, the Pfizer-BioNotech vaccine has to be kept at a very low temperature i.e. -70 ° C.
However, the Oxford-AstraZeneca vaccine produced in the UK can also be kept at a normal refrigerator temperature. Therefore, there are fewer challenges in transporting this vaccine. This vaccine can also be transported through cold chains of other drugs kept at low temperatures.
The end of this global pandemic is that the vaccine should be delivered first to the people who need it the most, and not to those who receive it first, who believe in buying it. The governments of rich countries have ordered millions of doses or doses of multiple vaccines simultaneously. But The World Health Organization has run a program called COVAX to provide vaccines to the poorest sections of the world's poorest and low-income countries.
Vaccine doses are taken from main centres to vaccination centres
When a vaccine reaches a country of its intended destination, its quality is tested at a specific location, rather than being delivered immediately. A special type of refrigerator is required to keep the vaccine safe.
Many countries have created special freezer farms to contain this vaccine. These are the warehouses where many deep freezers have been kept to keep the vaccine safe. When the testers approve a consignment of outside vaccines, they are transported to hospitals, clinics and other vaccination centres in special low-temperature vehicles.
Oftentimes the vaccines from the central vaccine hub are transported to regional centres before being transported to hospitals and clinics.
Vaccines are given to patients at vaccination centres.
Trained staff of vaccination centres are provided with a small consignment of a vaccine. In these centres, care is also taken to ensure that the vaccines brought to the patients are kept in the correct manner i.e. at the appropriate temperature.
Freezed vaccines are melted and diluted by bringing them to normal temperature before applying to patients. The real work begins when the vaccine is filled into the syringe and applied to the upper part of the patient's arm. After entering our body, this vaccine prepares our immune system to fight against the new coronavirus present anywhere in the body.
Vaccines are administered in the hope that those taking them are not the victims of the severe disease of Covid-19. It is only by keeping a close watch on those taking the vaccine over time, how long those who have been vaccinated are able to remain protected from the coronavirus.




0 Comments